How Does a Conductivity Meter Work
If youve got a new battery you will see around 15V on the display. This instrument is typically compact and handheld and features one or more probes.
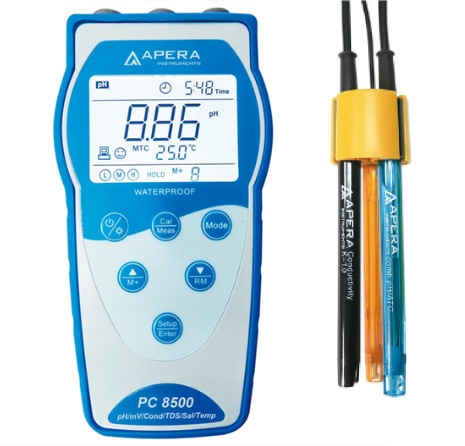
How Does A Ph And Conductivity Meter Work
So a Conductivity Meter is used to measure the volume of dissolved solids in a solution.
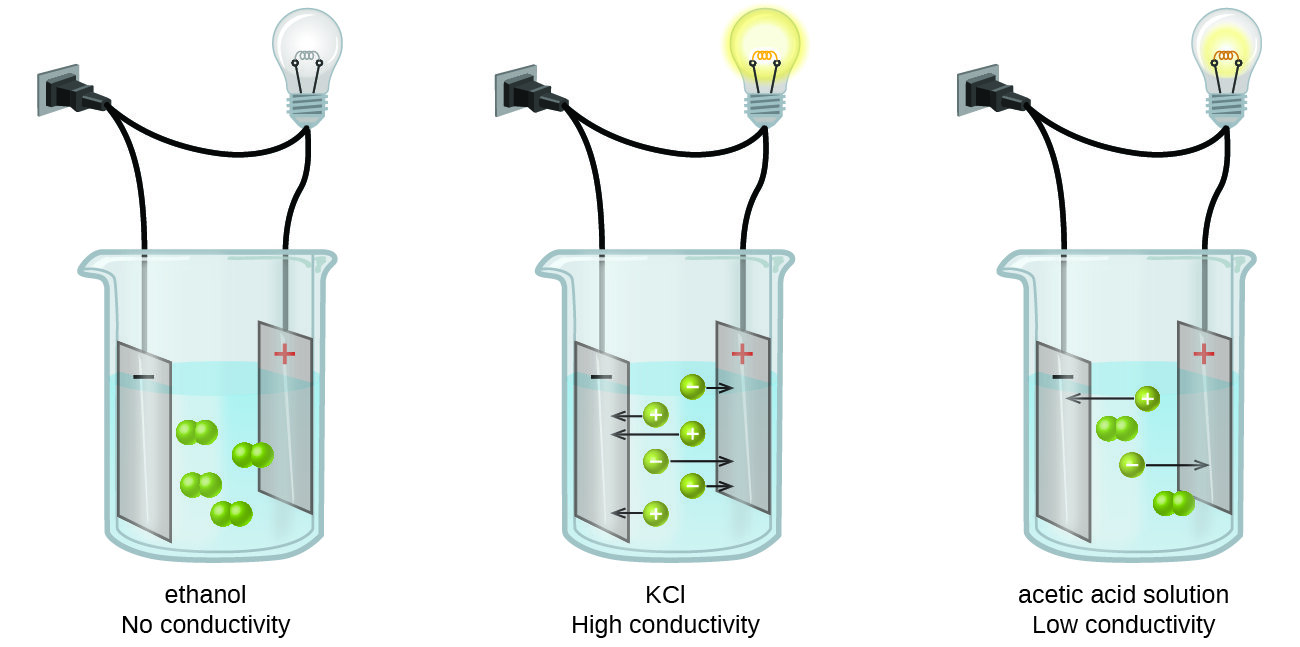
. 48 How Salinity is Measured Now more commonly with a conductivity meter Derived from conductivity and temperature measurements Practical Salinity Scale 1978 PSS-78 is unitless but values are sometimes reported in psu practical salinity units psu ppt. The relative concentration of ions within the solution will determine the conductivity or the resistivity of the sample. Measuring Conductivity To pass electric current through water a conductivity meter has two probes a small distance apart.
Generally the inductive method is better when the conductivity is high the liquid is corrosive or suspended. An electrical conductivity meter EC meter measures the electrical conductivity in a solution. How does a conductivity meter work.
This factor is usually 05. It has multiple applications in research and engineering with common usage in hydroponics aquaculture aquaponics and freshwater systems to monitor the amount of nutrients salts or impurities in the water. Conductivity of the electrolyte solution refers to the conductance of a 1 cm3 solution between two parallel electrodes spaced 1 cm apart.
However check device specifications the specific conversion factor used. Conductivity meter is an instrument and equipment suitable for precise measurement of various liquid media mainly used to accurately measure the conductivity of liquid media. The choice of which to use depends on the amount of conductivity the corrosiveness of the liquid and the amount of suspended solids.
Differences between Conductivity Salinity. When the electrodes are immersed in the sample the electrodes cause a current to pass through the sample solution. Where K 1ρ For conductivity J LA is called the electrode constant.
When choosing a conductivity meter make sure that it can measure the expected concentration of your sample. As you can see in the image the voltage for the batteries is being shown as 45V. The cell constant is the distance between the electrodes divided by the area of the electrodes.
G 1R 1ρ AL K 1J. Now press the probes against the positive and negative terminals of the AA battery. While this electrical current is applied to the solution the cations ions with a charge move to the negative electrode and the anions ions with a - charge move to the positive electrode.
These probes are attached to an instrument that displays the reading on a screen. When equipped with electrodes with corresponding constants the conductivity of high-purity water can be accurately measured. The units display Milli Siemens per centimetre mScm or Micro Siemens per cm μScm.
The greater the electric current the greater the number of charged particles present in the water. According to the above equation the conductance G of the conductor can be expressed as follows. The salinity meters software will produce a salinity measurement from the electrical conductivity reading by automatically apply a conversion factor.
It is also used to measure changes in wastewater processes in water treatment plants. These conductivity meters use alternating current frequencies and cell constant combinations that work best within a certain range so they minimize the effects of polarization. A conductivity meter works by emitting an electric charge through a probe that is placed in a solution.
MEASUREMENT OF CONDUCTIVITY There are two types of conductivity measurement. An electrical conductivity meter. Ions that have lost electrons are charged positively and ions that have gained electrons are charged negatively.
This is achieved because this equipment applies an electric field between two electrodes and performs a measurement of the electrical resistance in the dissolution. Connect the black probe to the batterys ground or - and the red probe to power or. The probes have sensors on the end that read the conductivity.
There is an online conductivity meter between acid adjustment system and membrane system monitoring the conductivity of the inlet pressure sensors at the entrance and exit of core type filter the filtration pressure and pressure sensors at the entrance and exit of high pressure pump and on the circulation pipe in the. When submerged in a sample the electrodes pass a current through the sample solution. Conductivity meter Conductivity is measured by conductance between two or four electrodes using the current method or potentiometric method.
A conductivity meter or conductivity meter is a device used to determine the electrical conductivity of the ions in a solution. A known amount of electricity is put down one probe and the amount that gets through to the other probe is measured. A conductance of a solution can be measured by applying an alternating electrical current to the two electrodes present in the probe.
To measure the electrical conductance of a solution one can use a conductivity meter. ProbeCell Constant of Conductivity Meter. Any increase or decrease in dissolving ions results in an increase or decrease in the electrical charge of the solution which the meter reads.
The effective thermal resistance of a system of two rods connected in series is given by RsR1R2 R s R 1 R 2 If these rods are of same physical dimensions and of thermal conductivities K1 and K2 then effective thermal conductivity of the system is KsK1K2K1K2. Electrical conductivity is measured by conductance between two or four electrodes using amperometric or potentiometric methods. The meter shows conductance as the inverse of a resistivity measurement in ohmscom which means conductivity is measured in mhoscm.
A basic introduction to the conductivity meter. K cm -1 Distance between electrodes cm Area of electrodes cm The smaller the cell constant the. The more particles in a solution and the more electrons being moved the more conductive a solution is.
K s K 1 K 2 K 1 K 2. A conductivity meter is a handheld device equipped with a probe to measure the amount of electrical current in a liquid solution. A conductivity meter measures the amount of electrical charge an aqueous solution can carry.
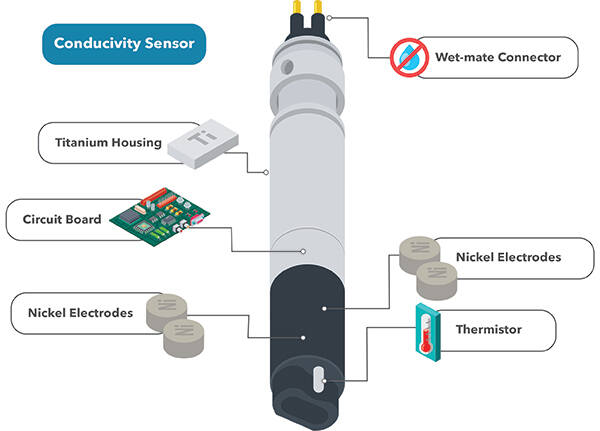
Conductivity In Water Salinity And Specific Conductance Measurement

Fully Automatic Self Calibrated Conductivity Measurement System Analog Devices

Conductivity Meter Holme Research Group Iowa State University

No comments for "How Does a Conductivity Meter Work"
Post a Comment